Characterizing tardigrades’ disordered protein to understand their resilience
by Bob Yirka , Phys.org SEM image of Milnesium tardigradum in active state. Credit: PLoS ONE 7(9): e45682. doi:10.1371/journal.pone.0045682
A team of researchers from the University of Grenoble Alpes, CNRS, CEA Institut de Biologie Structurale and one with the National (Taiwan) University has learned more about the unique protein made by tardigrades that allows them to survive in extremely hostile conditions. They’ve published their findings in Angewandte Chemie International Edition.
Tardigrades (also known as water bears) are micro-animals with an average length of just 0.5 mm. They have eight legs and have been found in every part of Earth’s biosphere. Prior research has shown that they are also able to survive in extremely hostile conditions—from heat conditions up to 150 degrees Celsius and cold to near absolute zero. They can survive exposure to dehydration, starvation, radiation and the vacuum of space with no protection. Prior research has also shown that when conditions are hostile, the tardigrades enter a dormant state that can last for an extraordinarily long time. When things improve, they wake up and resume their normal life. But how they maintain their dormant state is little understood. In this new effort, the researchers took a closer look to see if they could find the answer.
The researchers found that the disordered protein CAHS-8 produced by tardigrades forms into a type of gel during the tardigrade’s dormant state. Using NMR spectroscopy, they found that the protein was made of an “extended central helical domain” that was surrounded by disordered termini. When stressed, the protein becomes more concentrated and forms into long fibers. Eventually, the entire protein becomes nothing but fibers; the degree of its density is dependent on temperature. They also noted that a helical domain makes up the core of a fibrillar structure, while the termini continue to move about in the gel. Also, they found that soluble proteins could be stowed within cavities in the gel, allowing them to function during the dormant state. The researchers also found that the entire process simply reversed as the tardigrade emerged from its dormant state.
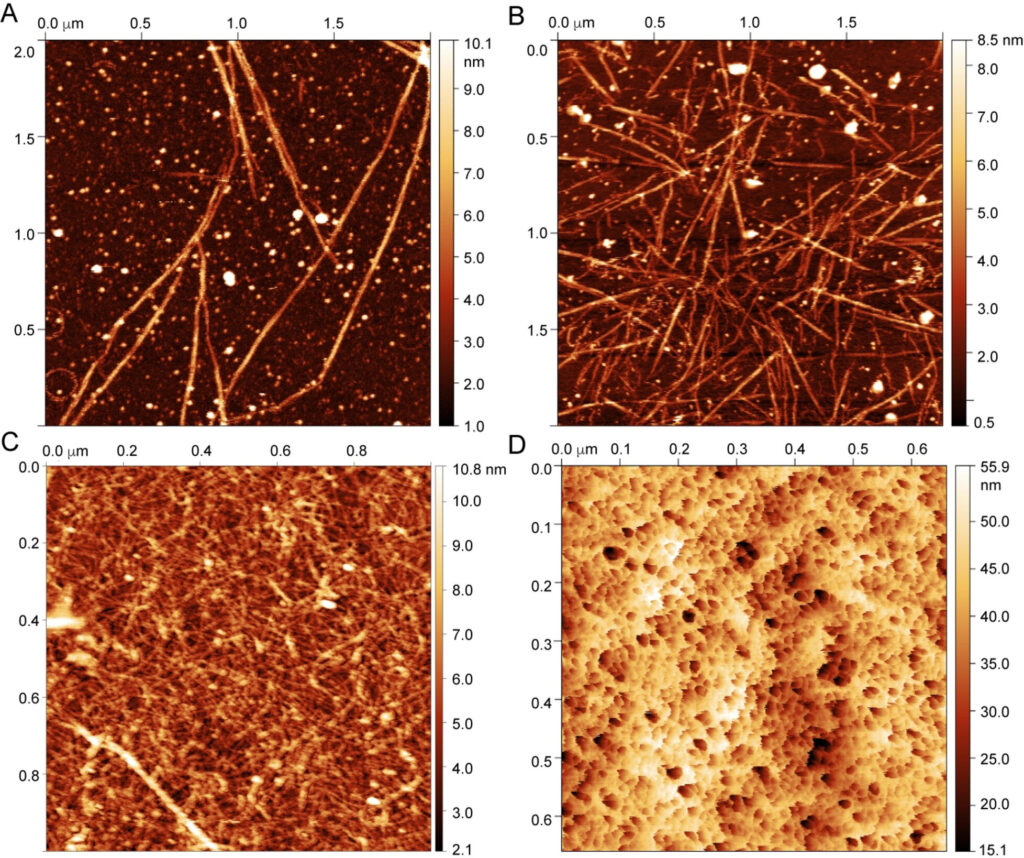
Different states of CAHS-8 imaged by AFM in the liquid (A,B) and air mode (C,D). A) Dissolving the gel on mica revealed fibrils and allowed a kinetic series to be recorded (see Figure 3). B) Allowing fibrillation to continue whilst the sample is drying shows an increasingly dense matrix of fibrils. C) After the kinetic series from (A) was recorded and the sample fully dehydrated, air imaging displays the entanglement of the fibrils forming a gel. D) Placing a 30 mg mL−1 gel on glass reveals porous structures, comprising cavities with diameters of tens of nanometers. Credit: DOI: 10.1002/anie.202109961
Reposted from: https://phys.org/news/2021-12-characterizing-tardigrades-disordered-protein-resilience.html